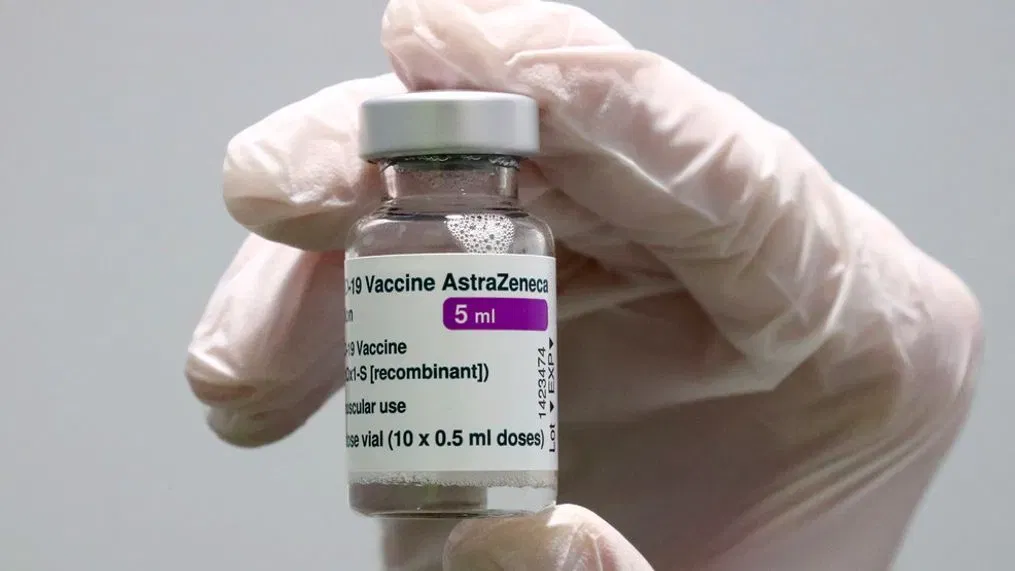
On Tuesday, pharmaceutical company AstraZeneca said it had initiated the withdrawal of its COVID-19 pandemic vaccine from the global market.
It noted that there was a surplus of available vaccines due to the multiple variants of the vaccine.

The company also said that it would stop selling the Vaxzevria vaccine in Europe.
According to various media reports, AstraZeneca, a multinational drug company, admitted in a court filing that one of its vaccines can cause side effects, such as blood clots.
The company applied to withdraw the vaccine on March 5, and it became effective on May 7.
The Telegraph first reported the development, and it was revealed that the withdrawal application was made public on May 6.
AstraZeneca’s vaccine for COVID-19 was manufactured by SII, which ceased production and supply of the product in December 2021.
The company shifted its focus to other vaccines and drugs after the pandemic’s sales declined.